Crack open any physiology textbook and chances are you’ll learn that after eating any normal meal, the release of insulin from the pancreas then signals the shutdown of the release of fatty acids from adipose (body fat) tissue and the increase of fatty acid uptake.
Because of this well-known role of insulin, one of the more puzzling explanations offered by some – including a few respected scientists and medical professionals — for weight gain is that elevated insulin is to blame because of its involvement in “fat storage”. In addition, they argue that the reason why a diet lower in carbohydrates works for weight loss is because of reduced levels of the peptide hormone.
It’s an easy conclusion to make. The logic goes that carbohydrates through their stimulation of insulin are fattening beyond their contribution of energy as kilocalories. It doesn’t matter how much you eat, so long as you avoid carbs to lose weight.
Another growing belief floating mainly around fitness circles is that it’s best to forego foods containing carbs when heading to the gym. It’s for fear that the carbs’ action on insulin will squash fat burning stimulated by exercise. Then again, some low-carb proponents have also argued, physical activity as a means to expend energy for weight management is pointless altogether. Again, carbs are really all that matter because of their action on insulin.
Where does all the extra energy from excess protein and fat go when overconsumed? And what about protein’s own effects in stimulating insulin or insulin’s role in promoting satiety? These questions are often overlooked or not easily answered by those that promote the “insulin is a fat storage hormone” proposition.
Out to help repair insulin’s reputation is obesity researcher Stephan Guyenet, Ph.D., of the University of Washington, who studies regulation of body fat by the brain. He downplays insulin as a primary regulator of long-term fat storage calling it a misrepresentation and oversimplification of what the hormone’s role really is. “There has been a lot of confusion about the role of insulin in the regulation of fat tissue,” Guyenet comments.
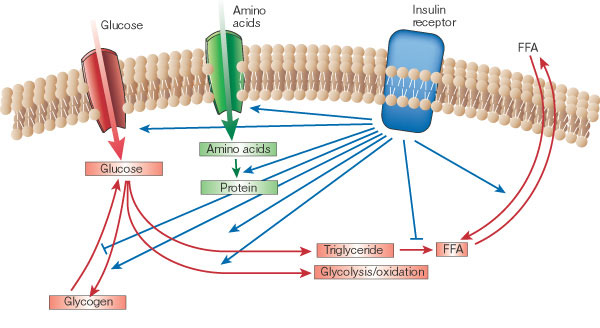
“Insulin is a critical coordinator of dynamic fatty acid flux on a meal-to-meal basis,” Guyenet clarifies. Through coordination, insulin is the main hormone that, essentially, tells the body what to do with the food energy just eaten. For example, when carbohydrate or protein is eaten, insulin directs the body to burn the carbohydrate or protein instead of using fat. And when mostly fat is eaten, the lack of an insulin response directs the body to burn the fat just eaten.
“Either way, you burn what you eat, and when that runs out, you go back to burning stored fat,” according to Guyenet. “This process is easily misinterpreted because one of insulin’s main functions following a meal is to shut down fat release from fat cells while the body burns carbohydrate and protein.
From Guyenet’s explanation, it sounds more as if insulin’s role is really that of a traffic cop— signaling where nutrients should go (carbs, protein, and fat) just after meals. Overall, however, insulin does not promote fat accumulation in the long run. Keep in mind that insulin also promotes the transport of glucose and amino acid into muscle for synthesis of glycogen and proteins after meals.
“At the end of the day, the total 24-hour ‘flux’ of fat in and out of fat cells does not appear to depend on these insulin spikes,” Guyenet comments.
Making friends with insulin through exercise
Exercise researcher John Ivy, Ph.D., of the University of Texas, is in agreement that insulin is an often-misunderstood hormone and also makes the point that insulin’s specific action following meals does not relate to long-term fat storage. “The most important thing is calories burned in a day,” he comments.
As previously shared on this blog, Ivy emphasizes that understanding how to make the best use of insulin and carbohydrate can be critical for long-term weight management. The main reason is because of their support to muscle maintenance and growth. Ivy reminds that insulin’s role extends well beyond its action on promoting fatty acid synthesis. The hormone also both prevents protein breakdown and boosts protein synthesis in muscle. Although protein by itself promotes insulin’s release (again, often overlooked by low-carb proponents), carbohydrates can boost both these effects.
Additionally, when carbohydrates are consumed during or after exercise, they can help fuel a better workout overall (as a fitness-model-nutrition-scientist colleague of mine once put it, “working out without eating some carbs makes you feel like @*$”). Carbohydrates also replenish glycogen for faster recovery and better workouts later. Finally, carbohydrates also help reduce levels of the exercise stress-induced adrenal hormone cortisol, which is implicated in protein catabolism, aiding muscle recovery.
The main consideration, Ivy explains, is that “one does not want to lose muscle.” Skeletal muscle can be a primary source of glucose disposal and energy consumption (with exercise) and can play a role in determining metabolic rate. But dieting alone often leads to both fat and muscle loss, which can lead to a reduced metabolic rate and an increasingly harder time continuing to lose weight. Without exercise, the chances of putting weight back on increases and that, in turn, leads to greater risk of insulin resistance. “Dieting alone is not the answer. To prevent muscle loss, one needs to exercise when dieting, which will accelerate fat loss,” he said.
He also emphasized the role of nutrient timing, or consuming nutrients at times that coincide with (e.g. carb/protein drinks) or directly after physical activity, to make the most of insulin. Directly after exercise is when muscles are most insulin sensitive increasing the likelihood that nutrients will be used for muscle protein and glycogen synthesis.
Diabetes researcher Joseph Henson, Ph.D., of University of Leicester, affirms that physical activity’s powerful effects on promoting insulin sensitivity makes it the “first line” of defense for prevention and treatment of insulin resistance. But while perhaps timing nutrients for after exercise may be best, he says that just getting any activity is what counts most. “A single bout of exercise can increase insulin sensitivity for at least 16 hours post exercise in healthy people as well as people with type 2 diabetes,” Henson comments.
On the other hand, the lack of activity, or general muscle contraction, Henson argues, can exacerbate insulin resistance and decrease insulin sensitivity, which are prerequisites for type 2 diabetes. Henson’s recent research suggests that probably more important than exercising regularly is simply avoiding staying sedentary for too long for cardiovascular health.
“We found that it was the amount of time that people spent sitting that had the biggest impact on glucose, triglycerides, and HDL cholesterol and not the level of exercise,” he said. “This just shows what a negative impact too much sitting can have on an individual’s health, independent of the level of exercise.”
With respect to the powerful effects of physical activity on insulin sensitivity and maintenance of skeletal muscle, it’s unclear why “insulin as a fat-storage hormone” proponents would argue against the use of exercise as an additional support for weight management. Of course, one could point out studies that have shown that exercise alone does little for long-term weight loss, but doesn’t that discredit further the idea that insulin is a long-term fat storage regulator?* “This is particularly ironic,” Guyenet explains, “since exercise is probably the most effective way to increase the insulin sensitivity of lean tissue. Exercise can also reduce fasting insulin to some extent.”
Why low carb really works for weight loss
If not because of reduced insulin, how then does a lower-carb diet really work for weight loss? Professor of regulation of food intake Margriet Westerterp-Plantenga, Ph.D., of Maastricht University Medical Centre, suggests that the reason low-carb works has nothing to do with carbohydrates at all, but everything to do with protein and its role on energy balance.
In a review paper last year, Westerterp-Plantega (along with colleagues Sofie Lemmens and Klaas Westerterp) wrote that controlled trials have shown that the answer is that it is the relatively higher protein of the diets including Atkins, South Beach Diet, Paleo, etc., and not the relatively lower carbohydrate content that has led to the success of these approaches for weight loss. The reason is that dietary protein acts on three “metabolic targets”: it increases satiety, stimulates energy expenditure, and spares fat-free muscle (helping to maintain resting energy expenditure).
Westerterp-Plantenga’s hypotheses are now supported further by a new study of which she was the lead author published earlier this year. The study compared two energy-restricted diets, one normal in protein (0.8g/kg/d) and one higher in protein (1.2g/kg/d) on 72 overweight and obese men and women. While both groups lost weight, as expected, the group that consumed more protein retained more muscle and had a higher resting metabolism (this was independent of physical activity).
Along with the study’s findings, which were published in the Journal of Nutrition, Westerterp-Plantenga points out that the protein in both diets facilitated satiety. The study evaluated satiety through measurement of concentration of appetite-regulating hormones glucagon-like peptide 1 (GLP1) and peptide YY 3-36 (PYY).
In addition, another recent study by Anita Belza and her colleagues, of the University of Copenhagen, evaluated different protein intakes on appetite-regulating hormones including GLP-1, PYY, and glucagon. The study was performed on 25 men in a three-way randomized, double blind manner that involved test meals with 30 percent energy from fat and normal protein (14 percent energy), medium-high protein (25 percent energy), and high protein (50 percent energy). The study, published in AJCN earlier this year, found that protein induced satiety in a dose-dependent manner.
These findings might explain why it has been observed in other previous studies, as reported by KJ Acheson, that “for every 1 percent increase in protein intake, replacing fat or carbs, observed decreases of energy intake range from 32 to 51 kilocalories.” That’s some satiety. In addition, he wrote that animal protein stimulates 24-hour energy expenditure slightly but significantly more than vegetable proteins (likely due to animal protein’s content of branched-chain amino acids and action on insulin).
All of this research support Guyenet’s assertions that fat regulation probably has a lot more to do with appetite than with insulin. “Insulin regulates fat cell function in a dynamic, meal-to-meal manner, but it is probably not a major regulator of total fat tissue size (despite its ability to alter body fatness in extreme circumstances like type 1 diabetes and insulin injections into the same spot every day),” he writes.
Leptin
In his final thoughts on what is really involved in controlling long-term fat storage, Guyenet adds, “The primary known regulator of body fatness is the hormone leptin.” How does leptin work to regulate fat storage? So far, what is known is that leptin inhibits food intake by affecting long-term appetite, although it doesn’t appear to have any effects on short-term satiety signals (as protein does).
Leptin acts specifically by signaling the hypothalamus that energy stores are sufficient. Notably, when leptin is absent (as in genetically altered mice and humans with rare mutations) it can lead to severe obesity. The effects of leptin in humans are not as well understood; however, obesity is thought to involve a resistance to the effects of leptin on appetite centers.
For more of Guyenet’s thoughts on carbs and insulin, you can head over to his blog. And, you can expect to hear more from Guyenet on the topic of leptin soon.
In the meantime, it’s safe to say that we should all lay off the naming and blaming of insulin for weight gain and obesity. Instead, it’s probably best to continuing to watch energy intake and expenditure, as well as making sure to include physical activity for improved muscle maintenance and insulin sensitivity, for long-term weight management.
*Note: Ivy suggests that to make the most of physical activity for weight loss, it’s important to get enough each day for its additional effects on appetite in the brain that could help diminish overeating. ”When physical activity declines below a threshold level of about 60 percent of resting metabolic rate, the relationship between caloric expenditure and appetite becomes uncoupled. The further one moves below this threshold level of caloric expenditure, the more one overeats,” he said.
References
Acheson KJ. Diets for body weight control and health: the potential of changing the macronutrient composition. Eur J Clin Nutr. 2012 doi: 10.1038/ejcn.2012.194.
Belza A, et al. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr. 2013 doi: 10.3945 / ajcn.112.047563
Henson et al. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia. doi: 10.1007/s00125-013-2845-9.
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001. doi: 10.1038/414799a
Soenen S, et al. Normal Protein Intake Is Required for Body Weight Loss and Weight Maintenance, and Elevated Protein Intake for Additional Preservation of Resting Energy Expenditure and Fat Free Mass. J Nutr. 2013. doi: 10.3945 / jn.112.167593
Westerterp-Plantenga MS, et al. Dietary protein – its role in satiety, energetics, weight loss and health. Br J Nutr. 2012 Aug;108 Suppl 2:S105-12. doi: 10.1017/S0007114512002589.
Wolfe R. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475-82.
I prefer high fat, moderate protein, low carbohydrate and minimal exercise without counting calories.
I put my 83 year old, 250 pound, sedentary (due to physical handicap), type 2 diabetic father-in-law on this type of diet several years ago. He lost 75 pounds, gained control of his blood glucose and was taken off all but one of his type-2 meds, which was reduced by half, after being on them for 20+ years.
Also, most “paleo” eaters would disagree with the characterization of their nutrient intake as “high protein” – a common misconception.
Cheers
Chuck,
I agree that “high protein” can be misleading. Perhaps as Westerterp-Plantega suggests, these dieta should be termed “relatively high protein” with energy-restriction (20-30% kcals from protein) — which really amounts to the same amount of protein as found in an energy-balanced diet (10-15% kcals from pro).
Thanks for sharing,
David
I also prefer trials which are not energy restricted – restricting individual nutrients is a better approach, it provides a clearer understanding as to how fat, protein and carbohydrates effect hunger and nutrient consumption.
Cheers
I may be oversimplifying here, but as a Type 1 diabetic, diagnosed in adulthood, I can attest to insulin’s role I fat storage. I had lost 65 lbs as part of a fitness plan prior to diagnosis and disease onset. When I got sick, I lost another 30 lbs, none of which returned until I began insulin therapy. I had not only lost most of my body fat, but I had begun to lose muscle mass. Insulin restored that and I began to appreciate it’s role (for good and ill) in fat storage/regulation.
Yes, very true, low carb and paleo diets are certainly not high in protein and do not claim to be so. Insulin is not to blame it is people eating far too much cheap carb foods. Who said low carb and paleo diets blamed Insulin itself…they blame excessive consumption of processed sugary junk foods.
This was a fair representation of the facts. I am of the opinion that protein intake should tend toward the higher side 1-2g/lb LBM, but as Chuck states above, if you exercise minimally a lower intake can suffice. Your note regarding Ivey’s statement regarding exercise and appetite coupling illustrates to me why exercise for most doesn’t yield appreciable results, especially in an obesigenic environment. 60% of RMR is a load for many, so IMO other factors regarding nutrition should be addressed such as satiation, satiety, palatability, food reward, and food variability. Exercise is healthful at appropriate doses but may only have an impact with weight maintenance. Well, I’m probably preaching to the choir here nevertheless, good article.
How is it that one “overeats”? The fact that this is the framing paradigm for this discussion reveals the limitations of your thinking on this matter.
I appreciate much of what Guyenet has to say on this matter, but post-meal insulin is one small aspect of the insulin-glucagon balancing act. While we know a great deal about insulin’s actions in terms of physiology and biochemistry, but we know very little about what is actually happening with real humans and their insulin activity over the course of a day–much less their lives. The range of “normal” for fasting insulin levels is tellingly wide, and we don’t even know if that’s the most useful measure (or is it insulin measured during OGTT or HOMA-IR or something else entirely).
However, to minimize the role of insulin in fat storage is disingenuous. Fat storage does not happen without insulin. Period. And fat loss will not happen if circulating insulin levels remain high–no matter how many “calories” (whatever is meant by that) you reduce, or for that matter, how many carbs. Period. How to best reduce circulating insulin levels remains a matter of debate, but there is no doubt that reducing carbohydrate and calories are usually both a part of that process in any studies done up to this point. But even then, this has more to do with fat loss than maintaining a normal healthy weight, which–you are correct in pointing out–is an intake issue.
What we are looking for is, I think, an eating pattern that is essentially self-regulating, given a person’s current environment, activity levels, and metabolic/physiologic needs. I mean, there’s a lot of food in my house right now, some of it very tasty, but I’m not eating all of it and I’m not having to exercise a great deal of willpower to refrain. Why is that?
Certainly, as our most important macronutrient, protein is crucial to self-regulation. And because most low-carb diets involve adequate protein–another concept we’ve yet to nail down (see the 2005 IOM macronutrient report section on it)–appetite may be better regulated. But LC diets do not usually involve much greater than normal protein intake, so how does that happen? What a LC diet will do, however, is rearrange protein in our daily intake patterns, so we may be less driven to keep trying to acquire adequate protein over the course of the day, thus accumulating calories we may not need metabolically because the foods that we are told to eat that are “healthy” and low-fat (whole grains, cereals, etc. especially) are low in protein. The insulin response to these low-protein foods is going to stimulate the storage of these calories as body fat.
Adele,
You bring up some interesting points. I especially am interested in relatively higher protein intake in meals (20-30%, for example) for self-regulation of appetite and, as you mention, how rearranging protein (over course of the day) can assist in reducing energy intake.
However, I will naturally disagree that downplaying insulin’s role is “disingenous” because, again, as these scientists have argued, people take “carbs-insulin cause fat storage” too far (you’re going to get an insulin response after meals anyway from protein or carbs). Energy balance (with all of its interesting nuances) appears to remain the framework by which to think about weight management.
David
Using percentage calories is an inappropriate way look at intake levels. Protein intake would thus vary considerably according to calorie intake when research research (Layman, Paddon-Jones, Wolfe, Volpi) have shown that protein intake is much more likely to have absolute thresholds that signal (or don’t) protein synthesis.
Which is one of the primary reasons that calories in/calories out is a very limiting way to look at weight management. There is far more to food than its caloric content. Food is information. The more we learn about nutrition, the more we realize that the signaling aspects of food have immediate and long-term consequences on metabolism (not just through hormones like insulin or leptin, but through transcription factors, neurotrophic factors, and epigenetic effects). A “calorie” of protein doesn’t exactly provide the 4 kcals of “energy” that we pretend it does anyway, right? What if the AAs don’t pass through acetyl-CoA into the TCA cycle, but instead are used for structure? Oops, guess I don’t have do that extra sit-up that “burns” those 4 calories. Seriously, when you get down to the cellular energetics of the matter, it all starts to look a little silly and when you keep that level of precision in place in your brain and climb back up the ladder to a physiological level, it looks even goofier.
It is always amusing to me that the folks most shackled to this approach are either career nutrition “experts” of one sort or another with an entire professional life invested in the concept, or young men for whom CICO might actually (sort of) apply. I’d like to see you and Stephan go through menopause and then tell me about calories in/calories out.
Adele,
OK, the menopause comment is hilarious and made me laugh. You make a good point that protein kcals not being exact, which is something I’ve written about beforehand. In fact, I’d be interested in your thoughts on my article from last February: “Calories aren’t right on labels and maybe that’s OK” (should you have the time to read it).
David
Hi Adele,
You asked “How is it that one “overeats”?” The definition of overeating is simple: consuming more energy than you expend. There are a number of controlled trials showing that when this happens, regardless of whether the excess comes from fat or carbohydrate, body fat accumulates. There are many factors that have been shown to cause energy intake to exceed energy needs for weight maintenance. Carbohydrate (per se) is not one of them, although specific highly palatable calorie-dense foods can be rich in carbohydrate and also promote overeating.
The fact that insulin is required for fat storage (i.e. an uncontrolled T1 diabetic will lose fat) does not mean that it regulates body fatness under normal circumstances. This is one of the many logical sleights of hand that underpin the insulin-obesity idea. There are many factors that are required for normal adipocyte function, but that doesn’t mean they all regulate body fatness in a normal physiological context.
Several controlled trials have demonstrated that fat loss occurs when a calorie deficit is present, regardless of insulin levels. Insulin appears to have no measurable impact on fat loss when calories are controlled. It’s always possible that there’s some small effect that has been below the detection threshold in previous studies, but the studies that have tested the hypothesis so far haven’t supported it.
You imply that energy balance no longer determines body fatness in post-menopausal women, and that your anecdotal experience demonstrates this. What about the commenter Diana (below), a post-menopausal woman whose experience is the opposite of yours? This is the problem with anecdotal evidence. It seems ironclad when you are the n=1.
I recognize that some people find low-carb diets to be effective for appetite control and fat loss (or maintenance), and if it works for them then that’s great. I agree that ideally you want to be able to maintain a favorable energy balance without having to bean count calories in/out. But there’s nothing more than circumstantial evidence supporting the idea that the effectiveness of LC diets in some people involves insulin. I currently can’t exclude the possibility that fat loss with LC diets involves changes in insulin action. But even if that’s true (again there’s no direct evidence to support it), it still doesn’t imply that insulin caused fat gain to begin with. I’m fairly confident at this point that elevated insulin isn’t a major contributor to obesity. There’s always the possibility that new findings will put this into question, but this is the majority view among scientists who know the most about insulin and obesity, and currently the evidence is on their side.
“There are a number of controlled trials showing that when this happens, regardless of whether the excess comes from fat or carbohydrate, body fat accumulates.”
Someone please explain to me all those pure carb overfeeding studies in which very little of the intake was converted to fat. I’m not taking sides here, I’m just sincerely confused. I think you know which studies I am referring to so I’m not supplying cites.
If more shoes go in my closet than go out of my closet then I have over-purchased shoes. That doesn’t tell anybody anything about why the shoes go in or why the shoes don’t come out. I agree that there are many factors that might cause me to either over-purchase or under-discard shoes (indulgent husband, Zappos, hyper-fashionability of shoes, etc), just as there are many factors which may cause someone to intake food beyond metabolic needs or that might prevent stored energy from being utilized. You’ve suggested that foods rich in carbohydrate may contribute to intake of food beyond metabolic needs, and surely you are not going to suggest (although perhaps you are) that those same carbohydrate foods will not cause an insulin response that prevents stored fat from being utilized.
I don’t think I’ve said that insulin “regulates” fat storage. As you’ve noted—and I agree—it is required for fat storage. In addition, as I know from clinical experience which of course is sadly merely a collection of anecdotal experience but I’ll get to that later, elevated insulin must come down before any measurable amounts of body fat can be utilized. I don’t know—and neither do you and neither does anyone else that matter—the full picture of how fat storage is regulated. Many folks have many pieces of the puzzle, but mostly the straight edges and not much of the messy middle.
“Several controlled trials have demonstrated that fat loss occurs when a calorie deficit is present, regardless of insulin levels.” This may be, but I don’t have any details about these studies so I can speak to them directly. It is possible that insulin levels in the subjects were low enough to allow for fat loss to occur; lowering caloric intake, which almost always includes some reduction in carbohydrate intake as well, may have gotten them to that point.
Granted I’m cranky and my forehead is wrinkly from treading over this subject repeatedly, so I fully understand how you might jump to that conclusion (and I forgive you), but I’m not actually post-menopausal, and therefore I wouldn’t imply that I have any personal anecdotal experience of the sort. I did imply, should you and David go through menopause and arrive intact on the far side, that one or both of you might. And your experience would not contradict the experience of someone else who found that post-menopausally, CICO works just fine thank you. People are different.
With that in mind, I really like anecdotal evidence. As far as I’m concerned evidence should be “ironclad” when n=1. I’ve found clinical experience to be a far more useful yardstick for measuring reality than the vast majority of what I learn from PubMed.
I’m not sure how you can hold the ideas that “insulin is required for fat storage” and “insulin doesn’t cause fat gain” both in your brain at the same time. It makes me sort of dizzy to do that (but I’m aging rapidly at this rate and perhaps now perimenopausal symptoms are to blame). I was also unaware that science is now a political system—and a democracy at that—and we can just put these things up for a vote. Just because the majority of scientists agree, doesn’t mean that same majority isn’t wrong. Hello? Germ theory?
What happens when you put a patient with T2DM on insulin? Ask any clinician who has done it. They get hungry, they eat, they gain weight, they have a really hard time losing it. It won’t happen to 100% of them, but it will happen to many. But that probably has nothing to do with the insulin, right?
It is not only possible that new findings will put the old CICO approach into question, that time has already arrived. It is already in question or we wouldn’t be having this discussion. What is not likely is that those questions will somehow evaporate through majority rule.
I’m not a Gary Taubes clone. There are many holes in the carbohydrate-insulin-obesity theory too. But CICO is the geocentric model—it explains some of reality, just not very much. The insulin theory is the heliocentric model. Better, but still explaining only part of reality. I am partial to looking forward to a “theory of relativity” for nutrition, not looking backward at a theory that explains less and less of reality all the time.
Hi Adele,
Your statement “elevated insulin must come down before any measurable amounts of body fat can be utilized” is simply not correct if you are referring to fasting insulin. Everyone taps into body fat every day at some point regardless of insulin level; that is just a normal part of energy flux in the human body. Obese people typically have a higher total rate of fatty acid release from adipose tissue than lean people despite higher insulin levels. Nothing, including insulin, is trapping fat inside the fat cells of obese people. I will refer you to Keith Frayn’s textbook “Metabolic Regulation” for a detailed review of human energy flux. I recommend not getting your information on lipid metabolism from popular authors and the Internet. When calories are restricted, even in the context of a high-carbohydrate diet, body fat is utilized. Insulin declines as a result of calorie restriction and fat loss in that case. The decline in fasting insulin is a consequence of calorie restriction.
The point of referring to the views of experienced researchers was to point out that the most knowledgeable people typically do not subscribe to the insulin-obesity idea. Of course reality isn’t determined by consensus, but nevertheless when the most knowledgeable people converge on an idea, it’s usually wise to take note. You said “Just because the majority of scientists agree, doesn’t mean that same majority isn’t wrong. Hello? Germ theory?” I could make the same argument about the fact that our bodies are made of cells. Just because scientists believe we’re made of cells, doesn’t make it true. It’s true because the evidence consistently supports it, and the fact that the best minds believe this “hypothesis” reflects the strength of this evidence.
Insulin doesn’t necessarily cause fat gain in diabetics. It is true that fast-acting insulins do often cause fat gain. This is partially because it is correcting a pre-existing insulin deficit, and also because it causes hypoglycemic episodes (a potent stimulus for food intake and fat gain). But the newer long-acting insulins like insulin detemir don’t cause fat gain. These cause fewer hypoglycemic episodes than fast-acting insulins. How does this square with the insulin-obesity idea?
The larger point however is that diabetes is a state of severe insulin dysregulation that is not necessarily applicable to common obesity, where insulin is doing its job just fine (evidence: blood glucose and free fatty acid levels are normal in most obese). As I said before, there is a difference between something that is required for a biological function, and something that regulates a biological function. This is a very important concept in endocrinology and the biological sciences in general. It is the reason why I can, as you said, “hold the ideas that “insulin is required for fat storage” and “insulin doesn’t cause fat gain” both in your brain at the same time”. These are perfectly compatible ideas if you understand the distinction between a factor that is necessary for a process, and a factor that regulates a process. A loss-of function condition like diabetes can’t demonstrate that insulin regulates adiposity or has any influence on it in the non-diabetic state. All it shows is that a certain quantity of insulin is required to maintain normal adipocyte function.
“The logic goes that carbohydrates through their stimulation of insulin are fattening beyond their contribution of energy as kilocalories. It doesn’t matter how much you eat, so long as you avoid carbs to lose weight.”
David, This is extremely unclear.
It confuses weight gain, weight stability, and weight loss, which are three very different things.
Carbs alone don’t make you fat, if you eat them in energy balance, with a low-fat, moderate protein diet.
But…when you get fat (as a result of eating too much carbs and fat in caloric excess) you have to take into account two things: caloric intake, and carbohydrate intake. Carbs do indeed inhibit fat burning (due to many factors); so does protein, but you’ve got to eat protein for (a) satiety and (b) LBM preservation.
Diana, I understand that the phrase confuses things. That was my point! Yet it’s the “logic” used by those who espouse the argument that insulin is the bad guy here. I agree with your other points. David
David,
Fair enough. My point is that merely restating the unclarity in their own words (they meaning the insulin and carb bashers) adds to the confusion.
You might want to devote a post to the fuel partitioning theory, which is admittedly a hard one to get around (in short: “carbs don’t make you fat, but you’ve got to restrict them to lose fat because of their lipogenic quality.”) That is if you haven’t already – have you?
Diana,
I’ll definitely give some thought to a post on fuel or nutrient partitioning. Anyone you would suggest as a credible source of information?
I’ll say, the more I learn, the more I’m convinced that you can’t really treat a sedentary and a moving organism the same way nutritionally. They are different animals altogether with different metabolisms.
Thanks for your comments.
David
I would say the phrase “are fattening beyond their contribution of energy as kilocalories” doesn’t reflect what the likes of Gary Taubes would say. He says all of the energy is accounted for in the laws of thermodynamics, but that in order for the fattening effect to happen, either 1) the consumption of carbohydrates either leads to an impulsive increase in more calories, or 2) that the insulin response would cause the metabolic rate to slow down significantly as to lead to a caloric surplus even while eating a starvation diet. This is due to “internal cellular starvation”. That is the nonsense he talks about in his lectures about the obese pima woman and the starving baby.
Sure, I’ll put it post on my reading list. And, since you’ve demonstrated (at least to me) that you might have a sense of humor, you can try my take on our current fixation on calories: http://wp.me/p29Lnc-4o
@Adele, ” I’d like to see you and Stephan go through menopause and then tell me about calories in/calories out.”
I did, I did, and it worked. I was in the low carb hell for years, was fooling myself about calories and it caused me misery and weight gain. I got real about calories and lost the weight I’d gained cycling fruitlessly from one LC to VCL attempt to another. I don’t give a frig what you call them: energy, heat, calories, I know damn well that certain combinations of food (usually but not always, carbs/fat) are bad for me. So I cut them out, and cut portions, and I lost weight.
Long story short: “I gained 50 pounds and I don’t know how” no longer holds water for me. You gained 50 pounds by eating more than your body needs, i.e., overeating. I am not going to get into a pointless exchange about the exact definition of overeating, or whether it exists. You can do that someone else.
Is it possible to consume more energy than you expend on a low carb (<50 g), moderate protein (<1 g / lb of lean mass), high fat diet? I think not. Are there any studies that prove otherwise?
Yes and yes.
Citations, please.
There has NEVER been a study done on overfeeding on a very low carb / ketogenic diet.
With that being said, I agree with Stephan, et. al. that the success of these diets has little to do with insulin or carbs per se. I think its very hard to overeat on these types of foods because they eliminate most of the problematic industrial foods.
Adele said that fat gain cannot happen without increasing insulin levels. How should we then interpret the many studies that shows that high insulin predicts LESS weight gain? (Swinburn et al. 1991, Valdez et al. 1994, Schwartz et al. 1995, Hoag, et al. 1995, Mayer-Davis et al. 2003, etc.)
Another example: In Meckling et al. (2004), they compared a low-fat diet (17,8 % fat, 600 kcal deficit) with a low-carb diet (15,4 % carbs, 763 kcal deficit) for 10 weeks. Only the low-carb group had their insulin levels reduced, still the low-fat group lost equal amounts of fat and retained more fat-free mass than the low-carb group. Doesn’t this indicate that there is no dose response between fasting insulin and fat loss?
It’s also interesting to see that in Dansinger et al. (2005) there were no significant differences in insulin levels between the Atkins diet groups (20 grams/d) and low-fat groups (10 % fat). And in Kasim-Karakas et al. (2000), the carb-intake increased from 53 % to 67 %, but insulin levels did not change. (http://ajcn.nutrition.org/content/71/6/1439.long)
Erik,
I wonder if this has anything to do with the fact that insulin is necessary to build all tissue, including bone and muscle. We have become obsessed with fat that we ignore everything else.
Quite interesting, Erik. Thanks for adding to the discussion and sharing the Kasim-Karakas et al link. I’ll review it.
10 Weeks! 600-763 kcal deficit! What a laugh. Try that for 10 years with no kcal restrictions, then get back to us.
I smoked a pack a day for 10 years. Didn’t get cancer, never had a heart attack…that was 40 years ago. Therefore, this conclusively demonstrates, beyond a doubt, that smoking does not cause cancer or heart disease.
Cheers
I agree with Chuck Currie. When I was diagnosed with T2 diabetes, I went LCHF and thought I could eat as much protein as I liked. But after 5 months my weight loss plateaued, and I had to increase my F:P:C ratio to around 84:10:6 to get it started again. I found during the plateau that it was extra protein that would send my weight up. I reduced the protein to 0.6g per lb of lean body mass, increasing the fat to compensate, and my weight loss started again. It has recently stopped again, but I’m close to my preferred weight, so I think the process is working as Volek and Phinney say it will. Now I’m more or less stationary, I am finding once again that a couple of days of more than 52g of protein will cause a 600-900g increase in my weight. I’m on no diabetes medication and have normal non-diabetic BG at all times, and a recent HbA1c of 5.0
I forgot to mention that I spend many hours a day just sitting. Exercise has never had any effect on my weight.
Once again, with feeling and in three-part harmony, I have not said that I think insulin “regulates” obesity. Maybe this is an issue of semantics. If insulin was not required for fat storage, then it couldn’t cause fat gain—got that. But since it is, it can? Or it might? If it might, then you can’t say with 100% certainty that it doesn’t. I’m not saying insulin is the be-all-and-end-all of fat storage; simply that it cannot be dismissed from the discussion just because some other alpha male in the science world (or out of it) has peed all over that tree already.
It is my understanding that the effect of insulin on hormone sensitive lipase is that fatty acids are blocked from being released from adipose tissue. I may be wrong about that, but I’ve gotten all the information that I have, paltry though it may be, from 4 semesters of biochemistry. I was a good student, so you’ll have to blame my instructors for teaching me the wrong stuff. Oh, then there’s clinical experience. I guess I would refer you to some clinical interactions and conversations with obese people who are having difficulty losing weight—preferably some who have insulin dysregulation issues but do not have frank diabetes. You can talk to these folks and measure whatever it is you need to measure to convince yourself that they are or not losing weight under whatever conditions you give them to lose weight under. Frequently the focus of mechanism-jousting (leptin insulin ghrelin, oh my!) discussions boil down to a bunch of snapshots from mouse/cell experiments that may or may not create a physiologically significant or—what is more important to me—practically useful understanding of what is happening in a human. Real people bodies sometimes seem particularly resistant to complying with what “should” be happening with them according to what we learned in books or according to our theories regarding a “normal physiological context.” That’s why Diana’s experiences with regard to menopause would be as valid as yours. When dealing with a particular individual, who gets to decide what is “normal”?
Btw, I think you are getting theories and beliefs a little mixed up. Right now, the calories-in, calories-out approach to weight regulation is—at least to me—a theory, i.e. a proposed explanation whose status is still conjectural and subject to experimentation. Ditto insulin and weight regulation and a long list of other things. A belief, on the other hand, is whatever you want it to be. We can test the theory that we are made up of cells, just as we can test the theory that at all that is needed for weight regulation is to have equal levels of caloric intake/expenditure (albeit perhaps not very accurately). We cannot test the belief that we are made up of cells, nor can we test the belief that at all that is needed for weight regulation is to have equal levels of caloric intake/expenditure because beliefs are not testable. They exist no matter what, supported by faith, tradition, and fairy dust.
@Erick, Nope. I did not say “that fat gain cannot happen without increasing insulin levels.” I said insulin was necessary for fat storage. I believe Stephan and I agree on that point. If you are going to quote me, quote me; don’t make up stuff–as a mother, I get plenty of that from home. It is possible that what we measure as “normal” levels of insulin is entirely sufficient for fat gain.
“just because some other alpha male in the science world (or out of it) has peed all over that tree already.” Ha! Adele, thanks for making my day and commenting on my blog. Please keep reading/commenting my posts. David
Too bad I don’t find this funny at all. Rather than blaming this on some imaginary alpha male, could it be the decades of research and thousands of published papers have led to the conclusion that insulin is not responsible for excess fat storage / obesity?
I’m sorry if I misquoted you. When I read “fat storage”, I assumed that implied increased fat mass.
Im a post menopausal woman..44% body fat mainly around tummy. Ive just cycled 2500km across europein 6 weeks in an attempt to lose my fat. I am still 44% body fat! Tell me how to lose that fat please.
Adele, Since you like anecdotal evidence, I am happy to give you some of the facts of my weight loss journey beginning age 55. In the interests of not wanting to hijack the thread, I’ll be brief. It was not fun. I went hungry. This is supposed to be un PC in the feminist wing of the blogosphere. Tough. I despised being overweight, and patriarchy has nothing to do with it.
I lost weight by going through 3-day bursts of very low calorie intake (protein fasts), followed by maintenance periods in which I ate anything I wanted but in small-moderate portions. I had never gone below 140 pounds since age 14, and when I did I was astonished and mentally liberated. Rather than distorting my “relationship with food”, creating control made it healthy for the first time since I was a small kid.
I’d be the first to admit that what got me through was motivation in capital letters – there is a psychological state in which the promise of a worthy goal powers you through temporary difficulties – including hunger.
But the long and the short of it was: I ate less. I lost weight. Physiologically, I do not understand why anyone can’t do this, as long as there isn’t some genuine metabolic problem. Psychologically, it’s a different matter, because a human being is much more than a collection of hormones.
@David – Thanks for providing such a lively forum. It’s been fun & I’ll keep reading. As I’ve said, I have the utmost respect for Stephan and his work. It all a part of the puzzle. I had the opportunity to meet him at a conference at Harvard. He brought his mother (which is just the most charming thing I think I’ve ever known a scientist to do), who is a lovely woman, and she and I had a chance to talk. He’s doing exciting work, and I think it will add important knowledge to body of science available on this topic. But all scientists get caught up in the bubble they are in. It’s an occupational hazard.
@ Diana – I am thrilled to hear that you found an approach that worked for you. It sounds like you took a rather idiosyncratic approach, which I applaud wholeheartedly.
You ate less and lost weight. This is what most people do on a low-carb diet, and we have some pretty decent explanations for this that don’t stray too far from CICO. Some people eat about the same amount as they do during weight maintenance or gain, yet will lose weight on a low-carb diet (in my clinical experience, these folks tend to be younger and are more likely to be male). We don’t really know why that is. I don’t claim any metabolic advantage, just ignorance. Maybe if they are less hungry, they are less likely to feel sad and cranky and there’s a metabolic lynchpin in there that we don’t know about. Maybe the change in macronutrients has less to do with carbs and more to do with protein or fat. There are people who can eat very little, exercise, and still not lose weight. I’ve worked with these people and I’ve been one of them. Nutrition experts like to assume we’re just “unaware” of our eating or “underreporting” or whatever. I prefer to suggest humbly that we don’t know everything there is to know about how any given individual’s body works, and it may be wiser to acknowledge that ignorance than to devalue someone’s personal experiences or question their veracity. I don’t really care whether CICO “wins” or the insulin theory does or some other theory (my bets, however, are with some other theory). My primary concern is for patients who have had their own experiences in relation to food and weight disregarded and their integrity questioned in favor of a prevailing theory (pick a theory, any theory). We need to find a better way.
Quite the interesting thread. From the original post I found it interesting that it flipped from you can’t control insulin with carbs, but “hey” you can control it with exercise and protein! I kinda thought it wasn’t about insulin? 🙂 Which then leads us, as always, back to CICO. Logic tends to bring us back to that conclusion, or as I suggest the “false dichotomy” in which we trap ourselves. Although I don’t go into great detail (depends on your definition) I do discuss the scientific logic we get stuck in on my blog here on wordpress and an article “Beyond the Calorie Model”. It’s simple really. As diane (or adele?) suggested food is about communication. So the theory I put out there is that it’s about stress management (stress is pretty complicated, using complexity, nonlinearity, systems and chaos models starting with neuroendocrine reflected geno-phenotypes as the sensitive “initial conditiions”). Leptin, sirtuins, mTOR these are all “supervisors” and central players in the “House analogy” I discuss along with insulin. Calories are important, they are the men, the energy, that show up to work. If you want the quickest and easiest way to save money on a job, they are the variable you take down to save money…. but that doesn’t always build the best house. Just because CICO is true doesn’t make it the solution, cause or central focus we need. THAT. is where our logic traps us. Insulin is also way too limiting. You have to look at all of the factors involved, singular assumptions or causes need to be left behind, which means moving beyond linear models. So realize we’ve been in the wrong scientific paradigm. Evaluate where that paradigm lead us astray. Look at where we are. And try to recalibrate to address the “stress” adaptations and how those diets; low carb, complex high carb, fats, proteins, mixes and interaction— adjust our “stress” systems. Which of course are complex ways in which we seek, store and adapt to resources. If anyone has time to read and would like elaboration I can do my best, but I think many of us are aware of all of these different pieces of evidence and diverse involvements of countless body/brain/gut systems, my post was my way to attempt to concisely put that evidence together, and make use of all of it in a way I think makes pretty simple common sense. We need real food and this why all of these various “diets” via macronutrient shifts or calorie restrictions create their impact.
“Beyond the Calorie Model”
http://wp.me/P39Q1V-AU
Carbohydrate creep – The scientific, government approved carbohydrate intake recommendation is 300+ grams per day. Can a person consume 300+ grams of carbohydrate per day without it creeping up over time without serious portion/calorie counting? I don’t think so.
Why have carbohydrate portions increased over the last 30 years, while protein and fat have remained relatively the same. What use to be a large popcorn and soda, is now the small.
I think the most important thing – which most people, especially you young people, either don’t know, don’t understand or discount – is: Dose Over Time. A one or two year, or even a five year, study, is not twenty, thirty, forty years of living. Sure, twenty-somethings can pound all the energy drinks they want, and a couple of six packs on the weekends and still have a six-pack, but when they hit forty, that six-pack starts looking more like they’re hiding a basketball under their shirt.
And don’t forget tooth decay – look at all the money and time we put into prevention and dentistry, without which we’d all be gumming our food by the time we hit forty. Growing up, it was drummed into our heads that candy rots your teeth – will, bread, pasta, soda, potatoes, pizza, sweet fruit, it’s all the same, and it rots your teeth. Dose over time.
Cheers
Hi Adele,
That’s right, I do remember meeting you at Harvard and having a nice conversation.
Insulin certainly does diminish fatty acid release from adipocytes in the post-prandial period, while the body oxidizes the carbohydrate and/or protein that was just eaten. As you said, this results from effects on adipocyte lipases, among other things. But elevated fasting insulin is not associated with a reduced rate of lipolysis. As body fatness (and fasting insulin with it) increases, on average the total body lipolysis rate increases. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3319738/
This is because 1) adipose tissue is larger, so there is more tissue to mediate lipolysis, and 2) adipose tissue becomes insulin resistant. The complications of obesity, e.g. diabetes, are probably at least partially related to insufficient insulin action in adipose tissue due to insulin resistance.
As David explained in his article, the fact that insulin reduces lipolysis and fatty acid oxidation in the post-prandial period does not imply that it influences 24 hr net fatty acid flux in/out of adipose tissue. When you eat fat without protein or carb, insulin doesn’t rise much and your body keeps burning fat. But you just ate fat too. Total body fat balance in that case depends on the amount of fat that entered your body, minus the amount you oxidized. Since eating fat doesn’t increase total energy expenditure relative to eating carbs, it follows that the energy content of what you ate (calories) is the important variable for determining energy balance, rather than the form in which the energy entered the body (roughly speaking). Insulin simply tells your body to oxidize the energy source you just ate. The default fuel is fat because we have a massive storage capacity for it, but if you’re eating as much fat as you’re oxidizing then you aren’t tapping into your fat reserves in a net sense.
That said, I recognize that LC diets can help with appetite control and fat loss in some people. The effects of LC on body fatness in controlled trials lasting more than 1 yr are unimpressive, but it’s not clear how much of that is due to reduced compliance over time. They may not be typical, but I do know a number of people who have gained weight on LC diets and lost it again when they went back to eating more carbohydrate and less fat. My view is that there’s a lot of individual variability in the response to diet and personal experimentation is in order. If LC works well for someone, then I’m certainly not going to argue with that approach (as long as they’re implementing it in a sensible way), but you can’t automatically attribute that effect to insulin when there are so many other changes happening in the body.
” . . . you can’t automatically attribute that effect to insulin when there are so many other changes happening in the body.” I couldn’t agree more, but I also would go on to say that you can’t automatically discount insulin effects either, since we know so little about how to consistently measure, report, compare it in humans (fasting insulin, for instance, may be the most relevant measure in some folks, but not in others–in still other folks, insulin may not be the issue, as you’ve suggested). I think the “cutting calories by cutting carbs” is a large part of the effect that we see. Is there more to than that? Maybe, maybe not. And maybe we’ll be able to answer that question, and maybe we won’t–or at least not for a long time. But then, you have a long career ahead of you to try to sort that out (and likely many other puzzles). I think your mother and I would agree that you have a great deal to contribute to this field & it will be a pleasure watching that work unfold.
I know that I’m often seen as an “advocate” for low carb diets. I’m not. I’m an advocate for getting essential nutritional requirements met in a way that best fits a person’s personal and health needs. For some, that means a low-carb diet, and for many, this approach has not been offered as an option–or has been opposed as an option–by healthcare professionals and media experts. I’m not opposed to low-fat diets, or to controlling calories, or to vegan/vegetarian diets–if you are healthy and happy, your choices are appropriate for you until your status changes. Just as the medical community is moving towards patient-centered care, we need to move towards people-centered public health nutrition–and that involves offering choices, being straightforward about the science (as much as is humanly possible in an all-too-human field), and honoring the integrity of individual experience. These are all sadly lacking in our current national dietary guidance.
Stephan,
“That said, I recognize that LC diets can help with appetite control and fat loss in some people.”
It goes further than that. How?
“The intake of dietary carbohydrates mainly has the effect of inhibiting fat oxidation while glucose oxidation is increased.”
ajcn.nutrition.org/content/59/3/682S.full.pdf
I am not saying carbohydrates MAKE you fat – they do not, overeating all macronutrients does – but if you are fat, they hinder your efforts to lose fat. Conclusion: cut calories by cutting carbs.
This conclusion seems to upset people on both sides of the aisle.
Im wondering, but will there be a follow-up post entitled “is it time to stop blaming overeating and sedentary behavior as causes of obesity” ?
heh, I don’t think so
But there should be a follow-up David. They (diet and exercise) may be part of the solution, but they are not the source of sole blame. So if one doesn’t think that question needs to be re-examined is the type of thinking that since we can prove the world is flat, damn i mean just lay on the ground should be proof enough, right? So we can prove the world is flat so why should we have explore that it could be round…is that type of thinking that is stagnating solutions.
Diana, “overeating all macronutrients (makes us fat)”, but if you are fat they (carbohydrates) hinder your efforts to lose fat. Conclusion: cut calories by cutting carbs”.
Carbs are our sources of energy, cutting them alters communication and burns more stored energy. Stephen made a comment that for “some” people cutting carbs works. (There is that wierd theory that it’s really about how much protein, but protein is a building tool, a raw material, so yes, that is a very important consituent, but it doesn’t negate, the carb reduction). So many diets are protein-carb or protein-fat, or protein-veg (no fat or carbs– like “SANE”). All will reduce weight, but we don’t know how successfully long-term or the long-term consequence (2nd generational) of that success. Losing weight, after the fact, doesn’t always represent the ‘best’ diet evolutionarily, only that we know how to tweak to get the body to do things.)
Cutting carbs or fats, our sources of energy and primary calories works, either actually, for both calorie reasons and communication reasons. Fats and carbs also work together to give us our “best” calorie sources. They “share” not only responsibility for keeping us working and alive, building and repairing, but also interact to “boost” and even balance each other. Eliminating one or the other can makes us stop that building and boost defenses and burn our stores. Burning, burning, burning shouldn’t be our goal, it’s just our current need to fix a problem. What is the problem? Obesity and diabetes and general malfunctioning of regulation (including alzheimer’s and the like). Is that caused by over-eating “all” macronutrients? Yes, but not really. These issues are caused by imbalance in our ability to build, repair and protect properly, they are caused, truly, by stress. What causes stress? Clearly too much fat and too many carbohydrates respectively and especially together. Especially in the absence of building tools and buffers (like the little gems we find in spices, herbs, fruits and veggies).
Amelioration of Obesity, Glucose Intolerance, and Oxidative Stress in High-Fat Diet and Low-Dose Streptozotocin-Induced Diabetic Rats by Combination Consisting of “Curcumin with Piperine and Quercetin”. http://www.hindawi.com/isrn/pharmacology/2012/957283/
Getting back to Stephen’s comment that “some” people cutting carbs works (for others fats, for others changing nutritional content, others fiber, others exercise, etc). There are patterns there that I have noticed. Again, I introduce it within my “House Analogy” and “East Coast/West Coast” folks, and the reasons I express the need to utilize Chaos Patterns (complexity physics) to understand those things. I, personally, and my personal theory for seeing patterns of who these individuals might be is through information processing reflected in neuroendocrine signally that creates brain patterning that utilizes particular resources (neurobiology of “personality”, but really personality is a reflection of coping up and downregulation of pain/stress and information handling, more like skill-sets reflected in brain and body physiology). So a loose example is like white matter (estrogen) utilizes more fat because of the mylineation, grey matter (testosterone) utilizes more glucose because of firing (this is not purely a sexual dimorphic, since it can be unisex, but it is built in this layering effect), so we see patterns of needs and the ways in which genetics, epigenetics, neuroendocrine, neurobiology, immune interact to create strengths, needs and susceptibilities (Gary Taubes sees the carb-issue weight gain in about 30% of the population, can we really come to a conclusion like sugar-insulin makes us fat even though this neglects 70% of the population??). And we then can gleen from studies these patterns in outcomes, drug actions, susceptibilities and expectations of outcomes utilizing these nonlinear, systems and chaos dynamics that reflect these needs and pattern of adaptations to the utilization of resources available culturally and historically.
Arguing about whether it is fat or sugar “causing” is like arguing if we fall or float of the the edge of the earth… the earth is round, it isn’t flat, it isn’t one or the other, it’s a much more complex dynamic and using linear dynamics (single direct causes, which is what most folks, determinely, continue to use is like the tail wagging the dog, and leaves out the story of whys and the glorious complications of human and neurophysiological ecosystems).
Great information and research ideas being shared it’s been very thought provoking education and reading.
Metabolic interactions between glucose and fatty acids in
humans. http://ajcn.nutrition.org/content/67/3/519S.full.pdf
Either we get fat because we eat too much, or we eat too much because we’re getting fat.
If it turns-out that fat storage precedes the increase in energy intake, then the insulin theory is almost certainly correct.
We don’t have any direct evidence either way, and until we do, this is all speculation.
In any case, as Adele has already pointed-out, you don’t so science by taking a poll.
No evidence, huh? *searches PubMed for “energy balance”; brings up 16979 papers*
Very Interesting discussion (Thanks to all of you for further confusing me 🙂 )
I have been a bit of a research WHORE for the LC & VLC way of eating after a not so great physical this year combined with a rather bad family track record for heart disease. In one year involving 2 job changes and working / traveling away from home and my HAPPY PLACE my TC & LDL took a 30% increase along with LDL pattern going to dense particle B pattern and already low HDL dropping even further to 34.One other note of reference is that my A1c dropped from 5.0 to 4.8. Fasting BG is ALWAYS at 100 + and i was gaining weight….
So of course the Dr. said Staten drugs we go…. I told her no and opted to take the VLC (less than 50 grams per day) and grain free way of eating for a 6 month test run. I lost 16 pounds in 6 weeks eating 2000+ calories with no exercise other than day to day living / working (mostly desk jockey). I did stall on the weight loss but i was not gaining. I was eating roughly 25 to 30 % protein and 60 to 70 % fat.i have reduced the protein to 20 % max and 75 to 80 % fat with lots of coconut oil because. I am seldom hungry even with modifying my meal window from a typical 12 hour (multiple small meal) to a 6 hour widow with just 2 large meals.
One thing that i have noticed in the discussion is that this study was once again a relatively short study at just 10 weeks. taking that the body takes roughly half of this time to adapt from carb to fat burning it is worthy of the curiosity to take the carb burners and compare to the properly adapted fat burners, with both calorie restricted and unrestricted for a 1 year test and compare information….
Just my thoughts
Ray
Good on you for not taking statins. Just curious: ” I lost 16 pounds in 6 weeks eating 2000+ calories….” How much did you weight beforehand? How many more calories than 2000 were you eating during your weight loss period? I’m not going to be coy: what I’m driving at is that you were eating enough to support 16 pounds more than you are eating now, you reduced caloric intake and lost weight. The carb reduction aided — I think it helped preserve lean body mass — but mainly it was reduction in overall caloric intake.
Best of luck, and continued success in controlling blood lipids.
yes i can see that my info was lacking some important details….
Actually i was eating at my actual tested BMR minus 500 calories or roughly 1700 calories. I use Myfitnesspal to track and was not eating low fat but more of a 30+gm P: 30 gm F: 35+ gm C and wasnt. I didnt eat a lot of crap carbs but i was not to concerned with grains but ate very little bread, rice, pasta, or potatoes. I ate a lot of beans as my main source of carbs
I set my calorie goal pretty close to my actual BMR and honestly as i added more fat to my diet i regularly exceeded the 2000 calories per day.
Sorry, I misunderstood. You created a calorie deficit of about 500 calories, and improved your macronutrient percentages. Now I understand, thanks.
Ray, so you increased your intake from 1700 to 2000 calories a day and lost 16 pounds?
started at 206 lb and now at 190 lb
and no i did not cut calories and actually increased them.
I only reduced total carbs to less than 50 grams per day and ALL grains starches and beans
Insulin’s demonization just doesn’t make good sense. Paleo people fret so much about insulin and carbohydrates to cause wonder if they work out much? I know, I know – for many, Paleo’s the latest panacea silver bullet diet. Do it and the world’s at rest. I call that allopathic paleo, where doctor prescribed pills and powders become the Paleo diet, all comforting sedentary ways of life that are the real culprit causes of what ails you.
Robb mentions our friend Dr John Ivy, former chair of Kinesiology & Health Education at UT Austin. John’s the pioneering scientist in the field of nutrient timing. Nutrient timing aims at optimizing peak performance in sports by optimizing metabolic response to training. His simple to make, simple to use nutrient timing drink is used post-workout to stimulate an insulin spike. That insulin spike signals insulin doing its job of getting amino acids and hormones into cells so they can do their job of cell repair and muscular growth. Athletes exhibit fine tuned, well working insulin receptors. John’s drink is a combination of whey concentrate, three simple sugars (I’ve used plain old Gatorade powder for nearly ten years, and some l-leucine, precursor to mTOR, the master hormone in cellular protein turnover sequencing). Tested subject who take the drink and have blood draws an hour later show low volume of testosterone. Good deal. The insulin spike facilitated a cascade of events drawing all available free testosterone to cells to be gainfully employed.
All and more is found in my August 2005 Iron Man Magazine feature story: Nutrient Timing & the Anabolic Switch (http://imbodybuilding.com/articles/nutrient-timinganabolic-switch/)
Everybody knows athletes in many sports use anabolic steroids. Most of us think that’s all they use. Since genetic splicing of pituitary cells in the mid 1980s, athletes gained a competitive edge resulting in as much as 20 pounds additional lean, hard muscle thanks to human growth hormone (HGH). Then in the 90s close to another 20 pounds of lean hard muscular development was facilitated by use of insulin. Insulin is more anabolic or more tissue building than steroids – along with IFG-1. Those big boys in a lot of sports regularly inject steroids, HGH, and insulin. Because they train hard, their insulin receptor sites are clean and working.
Instead of fretting about insulin sensitivity, work out hard and regularly, have fun, and that sensitivity grows naturally. Learn nutrient timing with simple carbos. Demonizing either is just plain foolish. If you’re going to invest time finding demons, the Sedentary Disease Syndrome is the one to go after. Inactivity messes up genes working properly; without exercise, the right switches aren’t turned on and the wrong ones prosper, resulting in slowly eroding metabolic meltdown. Loss of insulin sensitivity, type II diabetes, and inflammation are among sedentary symptoms putting life at risk.
Most Paleoist exercise recommendations are for newbies. The don’t tell you to outgrow infancy and childhood, gain a thriving robust adult state of genomic expression/wellness. Set your goal of four to six days of weekly training lasting 45 minutes sometimes, up to 2 hours other times. Surf the curve of strengths, extertions, all with focused mindfulness development.
“it’s unclear why “insulin as a fat-storage hormone” proponents would argue against the use of exercise as an additional support for weight management.”
To who? Are you unclear of the hypothesis having not properly researched it or are you familiar with it but just chose not to bring something of scientific merit to the table to refute it?
For the record the hypothesis (which is all the calorie in/out business is as well) is that the human body isn’t so easily fooled. That it if you put additional energy requirements on it, it will drive additional energy input and/or find other ways to conserve. Use your imagination on how leptin and the hypothalamus could, hark, possibly be involved with this.
What I’d like to see is the people who believe in the insulin hypothesis actually provide some evidence that the energy balance hypothesis is incorrect instead of just anecdotes on how they couldn’t lose weight eating 800 calories per day or some other nonsense that can easily be debunked by studies showing that people who have weight problems generally cannot accurately count calories.
I don’t mean to split hairs Ken O’Neil, because you’re on the right path mentioning “nutrient timing” and how important this internal communication is, mTOR being central, but mTor isn’t a hormone. Technically mTOR is a “serine/threonine protein kinase that regulates cell growth, cell proliferation, cell motility, cell survival, protein synthesis, and transcription. mTOR belongs to the phosphatidylinositol 3-kinase-related kinase protein family.” (wiki).
mTOR is a signalling pathway. A “hub” that takes in various and multidirectional aspects of information from the environment, stress and resources (including leucine as you mention which has a significant impact on its signalling (aka “i have protein, i can grow!”)) to make decisions about what it can and should be doing internally. This is why obesity (and chronic disorders) aren’t just about calories in and out, but rather what the calories and foods are doing, communicating, inbetween in and out. Insulin is part of that (but not the only–my preference is more a “stress hypothesis” mTOR’s a perfect example). To me, what calories, energy and resources, communicate to the system is what its about. But it’s also about many other forms of resources, duties and challenges the body must contend with and prepare for and even in the past have been communicated and programmed for (epigenetics). This is why it’s so confusing to mainstream which experimentally relies on “replication” science. In stress dynamics things don’t always happen the same way (you have to reduce and control to extreme refinement to get absolute replication, by then it’s good information but no longer applies to real life dynamics). The term science is actually, at its core, about predictability, not replication. And what we can predict with mTOR signalling is that there are many things that regulate what that pathway will decide to do. So different things, or many times different combinations will impact mTOR regulation similarly. To say that obesity is an issue of “sedentary syndrome” is a gross over-simplification and would be remiss to the complexity involved in the myriad of possible impacts to those same pathways (a solution doesn’t always signify cause). What’s true for one will not be true for another. This is why what is going on are stress mechanism and issues with the communication, not just a math issue of CICO. CICO isn’t wrong, but it needs to be folded into the larger whole. As far as “nutrient timing” as it applies to exercise, we could extrapolate the same concept that when 70% of the population is in metabolic distress the “ideal” diet may not be the a propo diet. And some of the macronutrient manipulations may be appropriate ways to regain balance for some.
More fun reading about mTOR for those interested.
From CellSignal.com:
mTORC1 integrates multiple signals reflecting the availability of growth factors, nutrients, or energy to promote either cellular growth when conditions are favorable or catabolic processes during stress or when conditions are unfavorable. Growth factors and hormones (e.g. insulin) signal to mTORC1 via Akt, which inactivates TSC2 to prevent inhibition of mTORC1. Alternatively, low ATP levels lead to the AMPK-dependent activation of TSC2 and phosphorylation of raptor to reduce mTORC1 signaling. Amino acid availability is signaled to mTORC1 via a pathway involving the Rag and Ragulator (LAMTOR1-3) proteins. Active mTORC1 has a number of downstream biological effects including translation of mRNA via the phosphorylation of downstream targets (4E-BP1 and p70 S6 Kinase), suppression of autophagy (Atg13, ULK1), ribosome biogenesis, and activation of transcription leading to mitochondrial metabolism or adipogenesis. The mTOR complex 2 (mTORC2) is composed of mTOR, Rictor, GβL, Sin1, PRR5/Protor-1, and Deptor and promotes cellular survival by activating Akt. mTORC2 also regulates cytoskeletal dynamics by activating PKCα and regulates ion transport and growth via SGK1 phosphorylation. Aberrant mTOR signaling is involved in many disease states including cancer, cardiovascular disease, and metabolic disorders.
Autophagy and cell growth – the yin and yang of nutrient responses. http://jcs.biologists.org/content/125/10/2359.full. A plethora of signaling molecules and pathways have been shown to have opposing effects on cell growth and autophagy, and results of recent functional screens on a genomic scale support the idea that these processes might represent mutually exclusive cell fates. This Commentary highlights recent findings that link autophagy and cell growth, and explores the mechanisms underlying these connections and their implications for cell physiology and survival. Autophagy and cell growth can inhibit one another through a variety of direct and indirect mechanisms, and can be independently regulated by common signaling pathways.
Stress and mTORture signaling. http://sabatinilab.wi.mit.edu/Sabatini%20papers/mTOR_stress_rev-Onc-2006.pdf
The existence of TOR homologs in unicellular organisms whose growth is affected by environmental factors, such as temperature, nutrients and osmolarity, suggests an ancient role for the TOR signaling network in the surveillance of stress conditions. Here, we will summarize recent advances in the TOR signaling field with special emphasis on how stress conditions impinge on insulin/insulin-like growth factor signaling/TOR signaling
SIRT1 Negatively Regulates the Mammalian Target of Rapamycin. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0009199. The IGF/mTOR pathway, which is modulated by nutrients, growth factors, energy status and cellular stress regulates aging in various organisms. SIRT1 is a NAD+ dependent deacetylase that is known to regulate caloric restriction mediated longevity in model organisms, and has also been linked to the insulin/IGF signaling pathway. Here we investigated the potential regulation of mTOR signaling by SIRT1 in response to nutrients and cellular stress. We demonstrate that SIRT1 deficiency results in elevated mTOR signaling, which is not abolished by stress conditions. The SIRT1 activator resveratrol reduces, whereas SIRT1 inhibitor nicotinamide enhances mTOR activity in a SIRT1 dependent manner. Furthermore, we demonstrate that SIRT1 interacts with TSC2, a component of the mTOR inhibitory-complex upstream to mTORC1, and regulates mTOR signaling in a TSC2 dependent manner. These results demonstrate that SIRT1 negatively regulates mTOR signaling potentially through the TSC1/2 complex.
Effect of dietary macronutrient composition on AMPK and SIRT1 expression and activity in human skeletal muscle.http://www.ncbi.nlm.nih.gov/pubmed/22674476. Under both conditions – overfeeding and caloric restriction – high fat/low carbohydrate (HF/LC) diet significantly increased phosphorylation of AMPK and deacetylation of PGC1α in skeletal muscle without affecting total amounts of AMPK, PGC1α, or SIRT 1. In contrast, low fat/high carbohydrate (LF/HC) hypocaloric diet reduced phosphorylation of AMPK and deacetylation of PGC1α. Our data indicate that a relative deficiency in carbohydrate intake or, albeit less likely, a relative excess of fat intake even in the absence of caloric deprivation is sufficient to activate the AMPK-SIRT 1-PGC1α energy-sensing cellular network in human skeletal muscle.
Oxidant Stress and Signal Transduction in the Nervous System with the PI 3-K, Akt, and mTOR Cascade. http://www.ncbi.nlm.nih.gov/pubmed/23203037. Oxidative stress impacts multiple systems of the body and can lead to some of the most devastating consequences in the nervous system especially during aging. Both acute and chronic neurodegenerative disorders such as diabetes mellitus, cerebral ischemia, trauma, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and tuberous sclerosis through programmed cell death pathways of apoptosis and autophagy can be the result of oxidant stress. Novel therapeutic avenues that focus upon the phosphoinositide 3-kinase (PI 3-K), Akt (protein kinase B), and the mammalian target of rapamycin (mTOR) cascade and related pathways offer exciting prospects to address the onset and potential reversal of neurodegenerative disorders. Effective clinical translation of these pathways into robust therapeutic strategies requires intimate knowledge of the complexity of these pathways and the ability of this cascade to influence biological outcome that can vary among disorders of the nervous system.
Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. http://www.ncbi.nlm.nih.gov/pubmed/20068142. Our findings demonstrate that mTORC1 promotes fat storage in mammalian cells by suppression of lipolysis and stimulation of de novo lipogenesis.
Fascinating article! Thank you for this compelling data, introducing me to Guyenet, and your excellent summary of his work. One thing though, and that is insulin resistance. Consider a body that has been living in a high-energy, high-insulin environment, over the course of twenty to thirty years. Ultimately, for that individual, cell receptors at the adipose tissue level, may not be as sensitive to insulin, because of long-term metabolic damage. This lack of sensitivity puts insulin back into the drivers seat, with regard to obesity, for said individual. This individual I describe, would be someone with insulin resistance, metabolic resistance, type 2 diabetes, etc. Evaluating someone with insulin resistance, equally to someone who has a healthy metabolism, is probably not an accurate description of the circumstances we find ourselves in, with regard to our obesity crisis. But again, fantastic data here. Thank you so much!
low carb diets work because of overall low cal intake,no one has ever been able to prove you can eat more then your bodys needs and NOT gain weight…
But how do you know what your body will need tomorrow? If you consume excess energy, why do you not just become more energetic? Why do high-carb, high-PUFA diets make people overeat? Why don’t those people then lose appetite or start fidgetting? Why do some people become energetic once fed, others lethargic?
This paper shows that, in a mouse model, the insulin/glucagon ratio (determined by dietary carbohydrate/protein) controls whether linoleic acid will have an adipogenic effect (high carb diet) or its opposite (high protein diet).
In other words, add an additional fat storage cue, control for glucagon, and the effect of insulin is clearly adipogenic.
http://www.jbc.org/content/283/11/7196.long
There are many great points in this article. It appears to offer an elegant solution that reconciles contradictory data about obesity, carbohydrate, insulin, and PUFAs.
A lot of good discussion about this article here:
http://forum.lowcarber.org/showthread.php?t=455297
A reblogué ceci sur Höf-day and commented:
Cool.
i need HELP, i get adrenal collapse and have an underactive thyroid With natural thyroxine and adrenal suppoet my life was saved My natural weight was 140 lbs
I am 72 now and i am now suffering with Diabetus as well. my first insulin made me put 40 lbs in a month , the doctor panic and changed the insulin. over the years my weight stablised at 186 lbs, then i became allergic to insulin. itching was driving me crazy.
So i was taken off and put on tablets, which worked well for a while and my weight went down to 150 lbs…..then i ended up in hospital with high sugars in a coma, so i was put back on insulin injections….guess what i have put on weight 35 ils in 3 months, around my middle. I look 6 months pregnant, I watch my diet and exercise. I fell 6 months ago and broke my shoulder, so the excess weight is causing pain in my back and shoulder. What can i do i am in a no win situation and i am getting fatter by the minuet. there must be a answer. help please, my body is different to mostand i need guidence. life is no fun anymore
One more variable I would like to throw into this discussion is nutrient deficiency and its effect. I’m posting this as a “food for thought” addition rather than any opinion one way or another. This may be a variable worth noting with regards to future research. For example, could deficiencies in vital nutrients such as magnesium (essential for normal glucose metabolism) be causing unnecessary variation in results? Could the incidental remedying of these deficiencies of subjects on various diets change an outcome?
In how many of these studies were participants screened pre/during/post for vital nutrient deficiency with a red blood cell test? Is Nutrient deficiency an irrelevant variable? Does the remedying of nutrient deficiency cause someone to lose body fat?
Here is an interesting study (among many) on magnesium deficiency –
http://www.ncbi.nlm.nih.gov/pubmed/24182515
Reblogged this on das leben ist ein märchen and commented:
Nur damit ich den Artikel nicht wieder verliere. Wer eine (s)Lowcarb oder eine Paleo-Diät macht, sollte das mal lesen: hat mir zu denken gegeben und etwas verwirrt. Ich weiss jetzt noch weniger warum meine Ernährungsweise funktioniert; vielleicht ist es ja doch reine kalorienreduzierte Diät, statt irgendwelchen “Biochemischen” Tricks, wie ich bisher immer dachte…
Hmm it seems like your blog ate my first comment (it was super long) so I guess I’ll just sum it up
what I wrote and say, I’m thoroughly enjoying your blog.
I as well am an aspiring blog writer but I’m still new to the whole thing.
Do you have any helpful hints for inexperienced blog writers?
I’d really appreciate it.
Going through decided that i would outdoor storage shed those types of extra pounds you want to restoration a quantity of intentions may well possible. Room a small number intentions is useful accepting helpful ways would you make a mistake at any time. Include less well known courses ?nstead of feeding some bigger courses each and every value more highly to use often the whole day.
http://kyleleoncustomizedfatlossreviewscam.blogspot.com/
Good article
Thanks for the information 😀
I have been researching the insulin-fat hypothesis and I agree with this article. It seems that people are viewing the issue quite linearly. “If insulin is high following a moderate-high carbohydrate meal (or protein meal) then one cannot mobilize fat in its presence and thus cannot burn it.”
If one goes beyond this singular thought process, we are can ask what happens when the steady state process of glucose uptake and and concurrent insulin production at rest is redirected to exercise. During moderate exercise glucose quickly begins to get shuttled out of the blood to the muscles etc where it can be used as energy to supply the exercise being undertaken. As shown here: http://www.ncbi.nlm.nih.gov/pubmed/23820621
Insulin also takes a dip as soon as exercise begins, therefore I’d guess that fat mobilization begins to happen again. In the study above this was shown after a meal with some glucose in it. So in a usual postprandial glucose graph, sugars take awhile t lower because these tests are usually done at rest. I’m guessing that if any kind of activity is added to the mix, the insulin hypothesis wouldn’t hold the same, but I’m just taking stabs at this. Why would we expect there to be fat mobilization and burning at rest. Wouldn’t it make sense that it would be stunted since at rest why would we need the energy from fat stores to be used, especially in the presence of calories from a meal we’ve just consumed? As activity rises, the calories from the meal are used at a faster rate and eventually other hormones are introduced into the mix to keep the blood sugar stabilized again. Apparently even walking can help:
http://www.ncbi.nlm.nih.gov/pubmed/20029518
Anyways, this is just a start, but I think people forget that other factors come into play and circumstances are important here.